PCCP for AI: The FDA’s Latest Guidance on AI in Medical Devices
On December 4, 2024, the Food and Drug Administration (FDA) announced a final guidance entitled “Marketing Submission Recommendations for a Predetermined Change Control Plan (PCCP) for Artificial Intelligence (AI)-Enabled Device Software Functions.” The FDA recognizes the iterative nature of AI-enabled device development and states that the guidance demonstrates their commitment to developing innovative approaches to the regulation of AI-enabled devices by providing recommendations on the information to include in a PCCP within a marketing submission for a device that includes one or more AI-enabled device software functions (AI-DSFs). The software changes covered by the PCCP are for AI-DSFs and could, for example, provide a pathway to incorporate additional real-world data to improve the AI performance.
The FDA recommends in the guidance that a PCCP for AI-enabled software describe the planned AI-DSF modifications, the associated methodology to develop, validate, and implement those modifications, and an assessment of the impact of those modifications. The FDA will review the PCCP as part of a marketing submission for a device to ensure the continued safety and effectiveness of the device without necessitating additional marketing submissions for implementing each modification described in the PCCP. A new filing is required when the conditions of the PCCP are not met, or the modifications result in an unapproved use of the device. The device manufacturer must utilize their quality system to document the implementation of each change successfully made in accordance with the scope and provisions described in the pre-approved PCCP.
Background
This guidance originated from comments received from a 2019 discussion paper entitled “Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD).” Further input was taken from “Good Machine Learning Practice for Medical Device Development: Guiding Principles” which was jointly published in October 2021 by the U.S. FDA, Health Canada, and United Kingdom’s Medicines and Healthcare products Regulatory Agency (MHRA). These ten GMLP guiding principles for AI/ML development are shown in the table below.
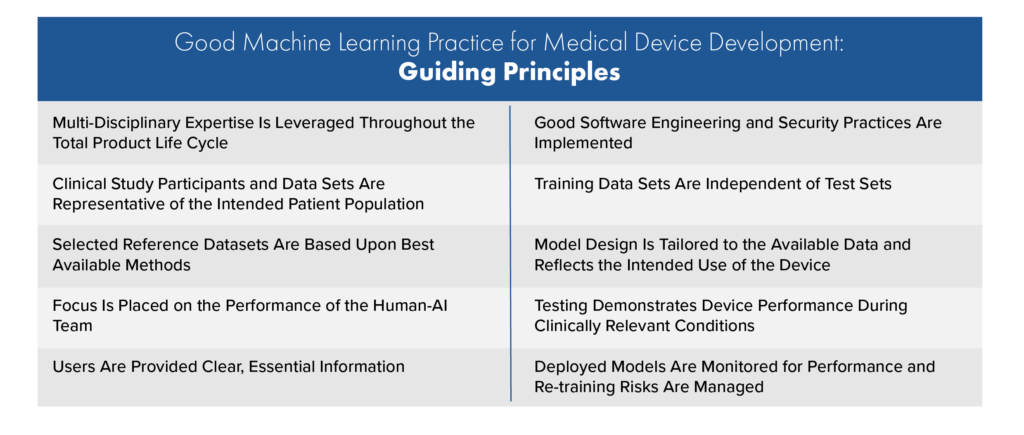
The PCCP approach comes from Section 515C of the FD&C Act (December 29, 2022) which has provisions regarding PCCPs for devices requiring premarket approval (PMA) or premarket notification (510(k)). While under the FD&C Act the FDA may approve or clear a PCCP for a variety of devices, this guidance provides recommendations specifically for PCCPs for AI-DSFs.
The FDA strongly encourages manufacturers to leverage the Q-Submission Program for obtaining FDA feedback on a proposed PCCP for an AI-DSF prior to submitting a marketing submission. The recommendations in the final guidance are based on the statutory authorities provided in the FD&C Act, including the provisions added by Food and Drug Omnibus Reform Act of 2022 (FDORA), but industry can use an alternative approach if it satisfies the requirements of the applicable statutes and regulations.
Overview of Guidance Content
The guidance has eight sections and two appendices.
Section I introduces the guidance. The guidance describes an approach that would often be least burdensome for manufacturers to support iterative improvement through modifications to an AI-DSF while continuing to provide a reasonable assurance of device safety and effectiveness. Later in the Guidance, the FDA states modifications that are appropriate for inclusion in a PCCP, such as those that are intended to maintain or improve the safety or effectiveness of the device. This emphasis on the device maintaining or improving safety and effectiveness after the changes are made is one of FDA’s primary concerns when evaluating the PCCP. As stated in the guidance:
“Specifically, this guidance provides recommendations on the information to include in a Predetermined Change Control Plan (PCCP) in a marketing submission for a device that includes one or more AI-DSFs. This guidance recommends that a PCCP describe the planned AI-DSF modifications, the associated methodology to develop, validate, and implement those modifications, and an assessment of the impact of those modifications. FDA reviews the PCCP as part of a marketing submission for a device to ensure the continued safety and effectiveness of the device without necessitating additional marketing submissions for implementing each modification described in the PCCP.”
Section II provides the background for the guidance. The background explains the history of the AI-DSF and PCCP discussion. This section also explains the statutory basis for the recommendations. For instance, it explains:
“In this guidance, we provide recommendations on the marketing submission content for a PCCP for an AI-DSF, which generally includes: 1) a detailed description of the specific, planned device modifications, which is referred to as the ‘Description of Modifications’; 2) the associated methodology to develop, validate, and implement those modifications in a manner that ensures the continued safety and effectiveness of the device across the intended use populations, which is referred to as the ‘Modification Protocol’; and 3) the assessment of the benefits and risks of the planned modifications and risk mitigations, which is referred to as the ‘Impact Assessment.’”
Section III explains the scope of the guidance. The guidance is applicable to all AI-enabled devices, including the subset of AI known as Machine Learning (ML). In fact, many of the recommendations are tailored to devices that incorporate ML in the AI-DSF. For the purposes of this guidance, a PCCP includes those device modifications that generally would otherwise require a new marketing submission.
“This guidance is applicable to AI-DSFs that the manufacturer intends to modify over time. This includes AI-DSFs for which modifications to the AI model are implemented automatically (i.e., for which the modifications are implemented automatically by software, also known as “continuous learning”), AI-DSFs for which modifications to the AI model are implemented manually (i.e., involving steps that require human input, action, review, and/or decision-making, and therefore are not implemented automatically), or a combination of both.”
Section IV of the guidance defines terms as they are used for purposes of this guidance. The terms are divided into three sets: 1) Software Functions, 2) Data Sets, and 3) Predetermined Change Control Plans (PCCP).
Section V of the guidance discusses the policy for PCCPs for AI-DSFs, including the components of a PCCP and where information about the PCCP should be included in the marketing submission for a device, and how to establish, implement, or modify a PCCP.
Sections VI-VIII of this guidance provide an overview of the recommended content for each component of a PCCP for an AI-DSF.
Section VI covers the Description of Modifications – The specifications for the characteristics and performance of the planned modifications to the device.
Section VII covers the Modification Protocol – The associated verification and validation testing activities that will support the planned modifications and acceptance criteria to assure the device remains safe and effective across the intended use populations. Four components are required in the Modification Protocol and should be traceable to the Description of Modifications: 1) Data management practices, 2) re-training practices, 3) performance evaluation, and 4) update procedures.
Section VIII covers the Impact Assessment – The assessment of the benefits and risks of implementing a PCCP, as well as the plan for risk mitigation.
The Appendices provide key information to help manufacturers implement the recommendations in this guidance. Appendix A includes detailed questions and considerations for the recommended content of a Modification Protocol in a PCCP for an AI-DSF, and Appendix B provides example scenarios for employing a PCCP for an AI-DSF.
What’s Next for PCCP for AI
To learn more about the final guidance, consider attending this FDA-hosted webinar on January 14: Final Guidance: Marketing Submission Recommendations for a Predetermined Change Control Plan for Artificial Intelligence-Enabled Device Software Functions.
Clarkston has related experience with machine learning solutions, data governance, integration of AI tools, assessing AI readiness, creating an AI agent, etc. For more details on these topics, click here.
We have extensive experience with assessing and implementing Quality Systems within the life sciences industry. Please reach out to us if you have any questions or need help ensuring your Quality System can handle the requirements originating from your PCCPs.
Subscribe to Clarkston's Insights



