Simplify The Supply Chain for Your Cell Therapy Products
Many new cell therapy products are currently in development that are classified as personalized medicine. One subset of that group is autologous cell therapy products which use the person’s own cells to generate their therapy.
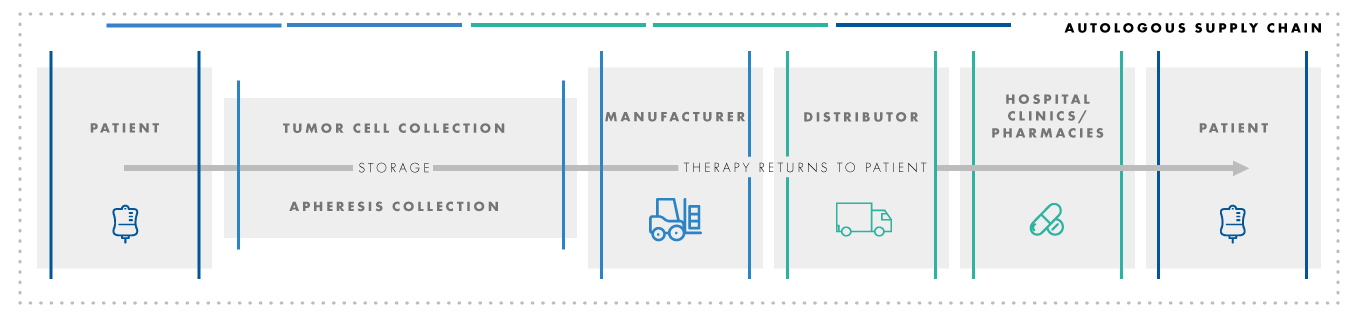
This process involves a complex and time-sensitive supply chain to obtain the cells from the patient, transport the cells to the manufacturing facility, modify the cells, transport the cells back to the patient, and reintroduce the cells into the patient’s body. Complexity is dramatically reduced when the cells are cryopreserved during storage and transport. Cryopreservation utilizes the vapor phase of liquid nitrogen to provide stable conditions (≤ -120°C) for storage and transport.
Cell Therapy Products
When the authors reviewed submissions for three recent cell therapy products, it was noted that the FDA had far fewer pre-approval questions for products that are cryopreserved. FDA’s concerns for use of refrigerated conditions (2-8°C) centered around cell stability during shipments of varying duration and temperature. Utilization of refrigerated cold-chain storage results in a situation where every step is time and temperature sensitive. Any delays or changes to the plan could cause a loss of product viability. The use of cryopreservation provides increased product stability and viability of cells, which makes the supply chain more adaptable to unforeseen circumstances.
Shipment tracking with temperature monitoring is standard in all cases but the shipment timing is critical for these unique cell therapy products due to the need to coordinate the many activities of the complex supply chain. A robust courier management process is critical to control the transport requirements. Any breakdown in the cold-chain process such as temperature monitoring or shipment duration needs to be monitored and responded to due to ripple effect in the remainder of the treatment process.
Clarkston Consulting’s Cell Therapy Orchestration Platform (CTOP) is built on the SAP S/4HANA platform and will provide you many benefits beyond the control of the supply chain. Reduce your worries about managing and tracking your couriers for cold-chain tracking, management of event timing, coordination of production and product management by adopting CTOP to manage your cell therapy products supply chain. Contact Clarkston Consulting for more information or a CTOP demonstration.
Learn More About Our Cell Therapy and Gene Therapy Consulting Services
Subscribe to Clarkston's Insights
Co-authored by Randy Jacobs.



