3 Operations Themes from the 2017 GPhA Annual Meeting
Last week, we were fortunate to attend this year’s GPhA Annual Meeting in Orlando, FL. As always, the event offered opportunities for networking and learning from the best of the generics industry. Over the course of this week, we’re sharing a three-part blog series recapping the key learnings from the event. These recaps will approach those learnings from three perspectives – strategy, technology, and operations. Below, our generics industry leader and operations expert Dmitri Serebrianik explores three key themes around operations from the 2017 GPhA Annual Meeting.
Like Joe and Brajinder, I was thrilled to take part in the final Generic Pharmaceutical Association Annual Meeting and (perhaps more importantly) the first Association for Accessible Medicine (AAM) Annual Meeting. If this year’s event is any indication, the generics industry is laser-focused on delivering their story AND their medicine to patients.

Generics companies have rallied around their cause and, for all intents and purposes, seem poised for a year of transformation and growth. As Joe discussed in his recap, generics manufacturers are building strategies around affordability, accessibility, and innovation. Actualizing those strategies requires significant operational change for many companies. Below, I’ve outlined 3 operational themes from the AAM event relative to those concepts.
Offering affordable medicine to patients requires a deep, hard look at your supply chain.
Every generics company present at the conference spoke with a single voice about one of the industry’s most important goals – making medication more affordable. With this in mind, each of the leaders cited a need to work closer with all of their partners and stakeholders. Doing so requires a comprehensive evaluation of your supply chain, from wholesalers and distributors to dispensers and service providers, for areas of improvements.
Realizing improvements to your supply chain passes on cost savings to your organization and ultimately and most importantly, to your patients. Some of the most common areas of untapped savings include:
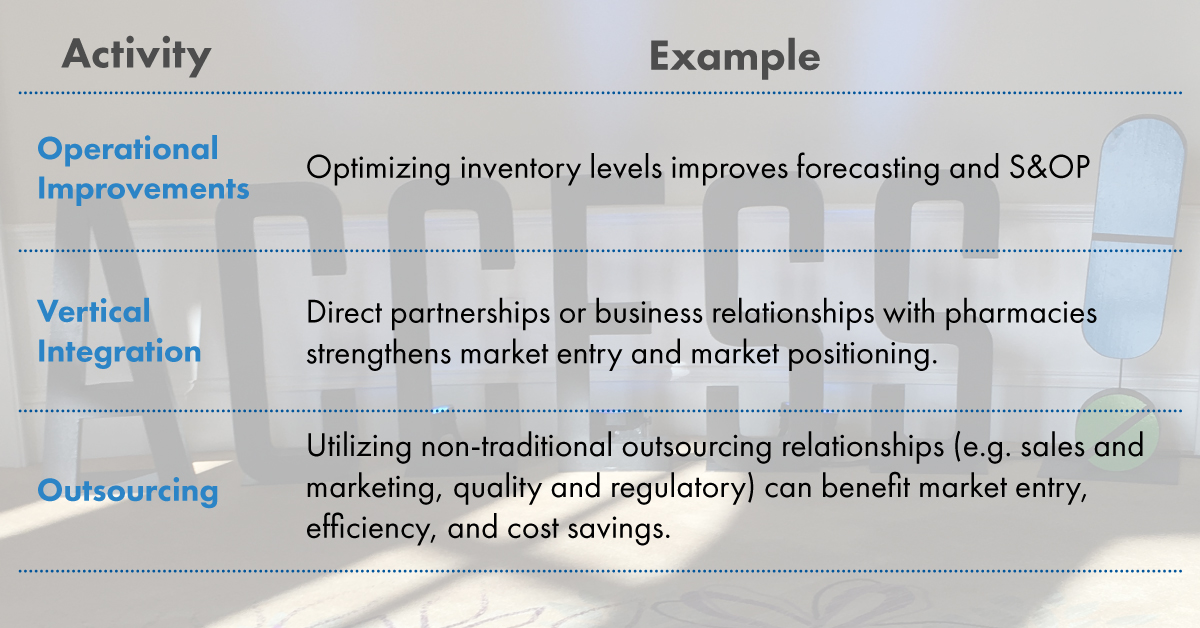
Improving patient access to medication boils down to one thing – reducing regulatory burden.
Throughout the event, it was clear that the generics industry and the FDA have the same goal – safe and effective treatment for patients. Achieving that goal in coordination and cooperation is the ultimate objective. Generics leaders referenced a need for better cooperation with the FDA and overall improvements to regulations. There was particular focus to GDUFA II.
With GDUFA expiring in September, manufacturers are looking to the updated program for a more efficient and effective Abbreviated New Drug Application (ANDA) review program. Improvements would reduce cycles to ANDA approval, increase the rate of approval, and ultimately grow access to generic drugs.
Asking for these improvements and preparing for them, as we all know, are two different things. Companies need to evaluate quality and regulatory operations to ensure preparedness for the new submission processes and FDA communications.
Innovation isn’t a sprint, it’s a marathon.
One message that particularly resonated with me through the AAM event was the need for continuous, near constant innovation. For generics manufacturers to deliver on their promise of providing affordable, accessible medicine, innovation has to be a priority at all times.
Fortunately, there are multiple avenues by which organizations can innovate from an operations perspective. Areas such as research and development, mergers, acquisitions, and divestitures, and go-to-market strategy are ripest for innovation. Some organizations might also find innovation in their product portfolio by pursuing biosimilars.
Innovative approaches to regulatory affairs will optimize the R&D process so generics manufacturers will be looking to tailor systems and processes to GDUFA and GDUFA II regulations.
As companies look to to reap benefits from M&A synergies and development of optimal product portfolios, they’ll need tangible services to help consolidate on:
- Technology Platforms and Computer Systems (SAP, QMS, and Manufacturing & Lab Systems),
- Process and Technology Integration,
- Data Migration, and
- Organization Transformation (Organizational Effectiveness, Communication, Change Management).
For more on the latest in generics and life sciences, don’t forget to subscribe to our insights below.



