GDUFA II Fees – What You Need to Know
The Generic Drug User Fee Amendment (GDUFA II) will go into effect on October 1, 2017. It will create a new fee for abbreviated new drug application (ANDA) holders to smooth FDA funding from unpredictable ANDA submission fees. GDUFA II will also remove the Prior Approval Supplement (PAS) fees and reduce active pharmaceutical ingredient (API) and finished dosage form (FDF) facility fees, making the funding provided by the ANDA holder fees even more important.
The FDA has created a tiered fee system to account for the size of the business. Small ANDA holders (1-5 approved ANDAs) will pay one-tenth of the large ANDA holder fee, medium ANDA holders (6-19 approved ANDAs) will pay four-tenths of the large ANDA holder fee, and large ANDA holders (20+ approved ANDAs) will pay the full ANDA holder fee. The full ANDA fee has yet to be determined, and it will depend on the number of small, medium, and large ANDA holders.

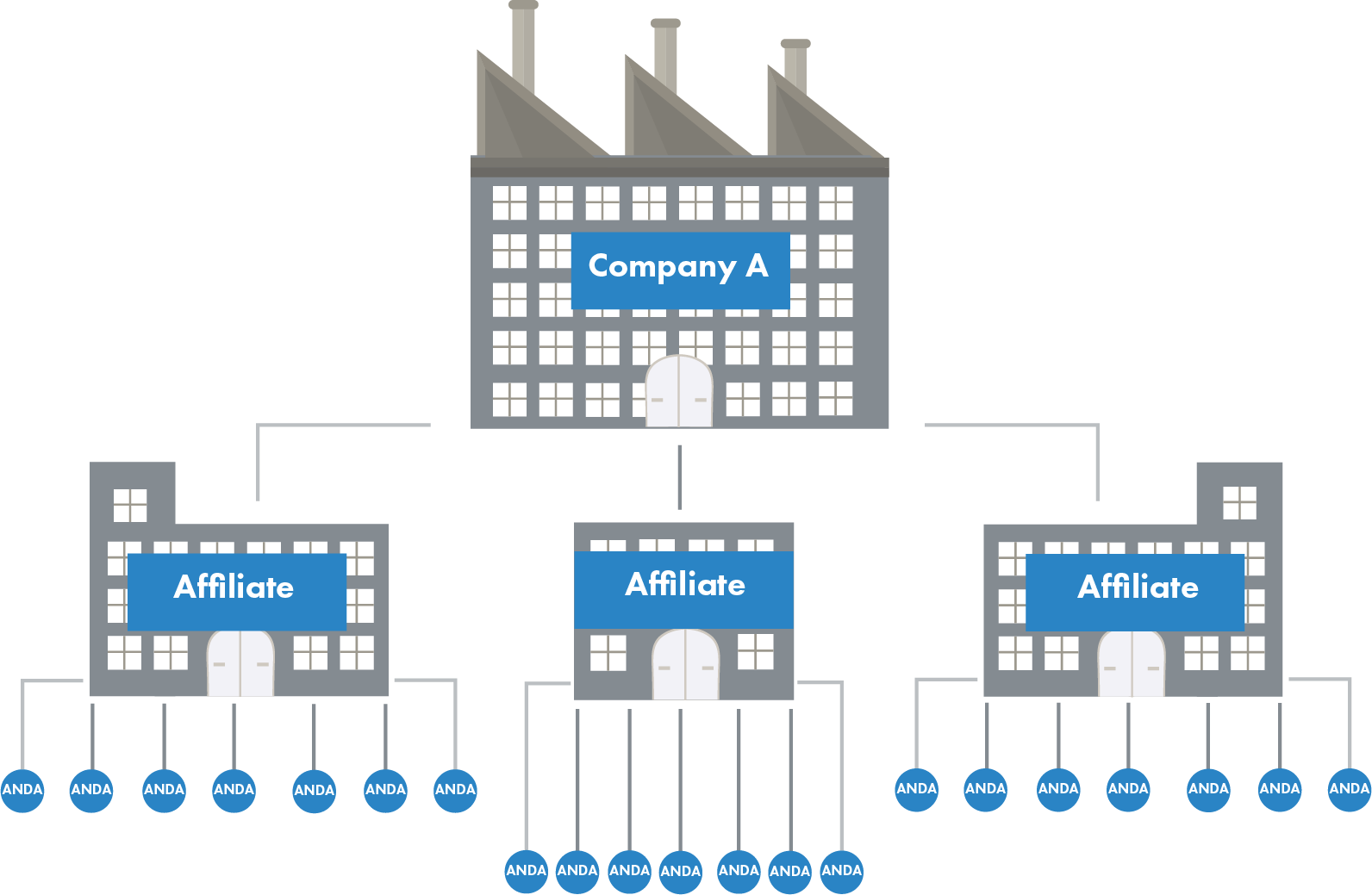
In the process of determining the ANDA holder fee, the FDA expects that affiliated companies will be grouped together and assessed a single ANDA holder fee instead of each affiliate being assessed a fee. For the FDA’s purposes, an affiliate is “a business entity that has a relationship with a second business entity if, directly or indirectly, one business entity controls, or has the power to control, the other business entity; or a third party controls, or has the power to control, both of the business entities.”
As part of planning for the 2018 ANDA holder fee, the FDA has gathered and released an inventory of every approved ANDA and the company holding each ANDA as of March 10, 2017. The agency has requested that all ANDA holders review and comment on the inventory to ensure both accuracy and that ANDAs held by affiliated companies are claimed appropriately. For example, Company A holds a total of 21 ANDAs, but they are spread among 3 affiliates, each with 7 ANDAs. In this example, Company A would end up paying 3 separate medium ANDA holder fees, which would exceed the cost of the large ANDA holder fee that Company A would pay if it claimed all the ANDAs under one affiliate.
GDUFA II Estimated Fees
Based on the current ANDA inventory, the large ANDA holder fee is estimated to be $1,344,436, the medium ANDA holder fee to be $537,734, and the small ANDA holder fee to be $134,444. In the previous example, Company A would end up paying $1,613,202, an overage of $268,766 that they would not need to pay if all affiliated ANDAs were claimed. Be aware that as affiliated business entities are claimed and grouped appropriately, the estimated fees will change.
The FDA must publish the GDUFA II ANDA holder fees by August 2017. As of May 22, 2017, the FDA ANDA inventory contains 1,963 unclaimed ANDAs held by 378 business entities. Many of these are held by affiliated business entities (an example would be Valeant Pharmaceuticals, listed in the inventory as 4 distinct business entities). All ANDA holders should review the inventory and communicate any business entity affiliations to ensure that they are not paying excess ANDA holder fees for FY 2018.
At the time of the latest inventory update, the FDA gave companies 60 days to review and comment. Time is running out! Act now to ensure your company is not overcharged!
Instructions for using the ANDA inventory and submitting claims can be found here.
If you found this information interesting, subscribe to our blog to receive the latest insights:
Subscribe to Clarkston's Insights
Co-author and contributions by Mike Lanewala.



