Data Integrity Problems Break Trust, Part 3
In part one of this series, I talked about data integrity at its most basic level – including what it is and why it matters. Part two covered the impacts that poor data integrity can have to both your quality systems and your bottom-line.
Today, I’m highlighting some of the foundational elements for mitigating data integrity issues. What you’ll find is that data integrity issues are complex…and getting more complex every day. What I’ve found on project after project is that data integrity gaps are, more times than not, completely involuntary and just as completely unknown, making their diagnosis and remediation quite difficult.
Assessment
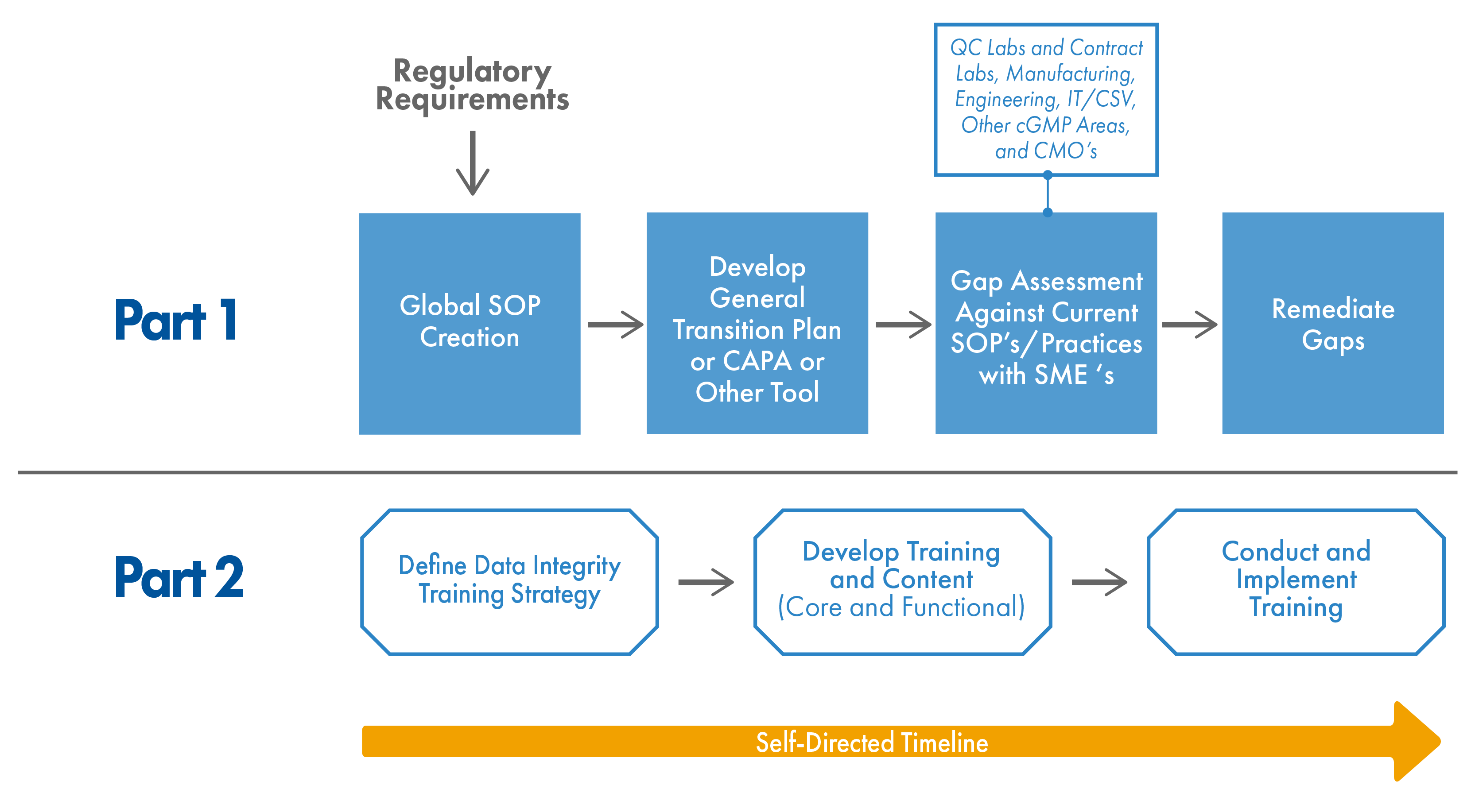
Overcoming data integrity issues starts by: a) knowing the issues exist, and b) knowing where they are. That might seem incredibly obvious but it ends up becoming a substantial challenge for some organizations. Unfortunately, there’s no one-size-fits-all (or even a one-size-fits-most) approach to performing these audits as they must account for the scope and size of your systems, processes, and organization. We suggest starting with an assessment checklist template that can be customized and adapted to your organization.
Upper Management Commitment
The success of a data integrity initiative can be made or broken by the commitment of upper management. One thing that comes up a lot in our data integrity projects is the concept of a “culture of strong data.” Building and strengthening that culture of good data integrity principles requires buy-in and near constant championing from upper management. That’s not to say that it’s an overly intensive exercise – something as simple as a few lines in your organization’s quality manual is a good start. From there, a corporate SOP can serve as a solid foundation that, when coupled with a transition plan, serves as a road map to identifying and remediating any data integrity weaknesses.
Methodology
At the heart of any mitigation strategy is an evidence-based and scalable methodology. A methodology for ensuring data integrity should include multiple elements, including but not limited to:
- Defining and enforcing appropriate data integrity policies and practices
- Ensuring data integrity policies are fully compliant with regulations from regulatory agencies
- Raising awareness of data integrity within your organization and ensuring employees are properly trained on data integrity concepts
- Establishing a data integrity initiative
- Perform periodic internal data integrity audit
Mitigating Data Integrity Issues: Stay Informed
Go straight to the source – the FDA regularly finds, cites, and publicizes common data integrity deficiencies, including inspection trends. Consistent tracking, analysis, and application of inspection findings can bolster your own data and ensure your priorities are in line with those of the FDA. The FDA also released a guidance document in April 2016 to give drug manufacturers an understanding of the FDA’s perspective relative to data integrity.
With parts one, two, and three now complete, you have a foolproof, completely exhaustive, and flawless understanding of all things data integrity….right? Absolutely not, unfortunately. The true challenge of data integrity rests in its ever-evolving nature – data is growing, spreading, and being leveraged in new and different (and exciting!) ways all the time. That said, what I’ve provided here, though not all-encompassing, does provide you with the foundational understanding you need to build, repair, or enhance the integrity of your data.
I thought I’d close this series by offering up a few questions for you or your team to consider as you think through your own approach to data integrity.
- How do your documented procedures address review of audit trails?
- Do you have different data integrity policies for your quality team vs. the rest of your organization?
- How do you ensure that all metadata for both paper and electronic systems are maintained throughout the document retention period?
- How does your executive team support data integrity initiatives?
- Does your organization offer regular and consistent process and system training which includes data integrity concepts?
- Is the source of your data well documented and understood?
- Do you know what’s coming from regulatory agencies related to data integrity that may impact your industry?
Please feel free to contact me using the information at the top of this page to discuss these questions or anything to do with data integrity. I’m always interested in learning more about how organizations of all industries and sizes are confronting data integrity issues in our increasingly data-dependent world.