Gene and Cell Therapy Product Documents to Drive Decision-Making
A new job at a start-up in the Cell and Gene Therapy (CGT) area is exciting, especially when funding is solid with scientifically sound technology. Imagine that you’re on the leadership team at this new CGT start-up, and you’re going to be involved with the Investigational New Drug Application (IND) filing. Your background isn’t in CGT, and all the business processes you’ve been using your entire career don’t seem applicable to a technology you don’t understand … yet. The leadership team needs to bring innovative technology – and the first candidate with at least one potential indication – to an IND filing. The entire company will be looking at the senior leadership team to, well, lead. Where do you even start? This is where gene and cell therapy product documents come in handy to drive decision-making.
An Investigational New Drug Application (IND) is a request for the Food and Drug Administration (FDA) authorization to administer an investigational drug to humans through clinical trials and to ship experimental drugs across state lines.
CGT research and development is different than that of small molecule (chemical synthesis) or large molecule (biologics). To use an overused analogy, we are building the plane while we’re flying it. This may be true, but the business concepts are the same regardless of the specific area in life sciences.
As a pre-clinical startup, the company is early in its evolution, the technology platform is being developed, formulation is being changed as the latest information learned is leveraged, and – at the same time – the analytical team is figuring out how to analyze key quality attributes. Moreover, team members have joined the company to learn about CGT. In such a dynamic environment, how do we stay focused and ensure timelines are achieved?
Product Documents That Drive Decision-Making
There are a number of documents that are vital to the product lifecycle, regardless of the type of product, within life sciences. These documents ensure the entire team understands what is to be delivered and what success looks like. These documents are especially important in the early stage of a product’s lifecycle, programs are moving fast toward milestones with tight timelines. Having goals and objectives formally documented ensures team alignment and supports project decision making. Let’s look at a couple of them that establish the framework for execution:
Corporate Goals: The company should have its driving principles documented. An inspiring mission statement is great, but without the direction of goals, it’s more of a gesture than something to achieve. Documented corporate goals tell us where we want to go and what the Board of Directors have agreed upon as important from a product perspective. There are usually not too many of them with objectives to achieve each of the goals, and the objectives typically cross several functional areas. Ultimately, these goals should drive the CTP and TPP, which we address below.
For this new company, one of the Corporate Goals for this year may be to nominate a new candidate, another could be process lock of the formulation for the lead candidate.
If you are the head of a functional area in Technical Operations that supports a CMC (Chemistry, Manufacturing, Control) team, the objectives to support the corporate goals need to be further defined. Using SMART goals will ensure the team has a clear understanding of what they are working toward, how progress will be measured, and how they relate to the corporate goals.
Image Adapted from the Indeed Career Guide
Product Lifecycle: Candidate to Control
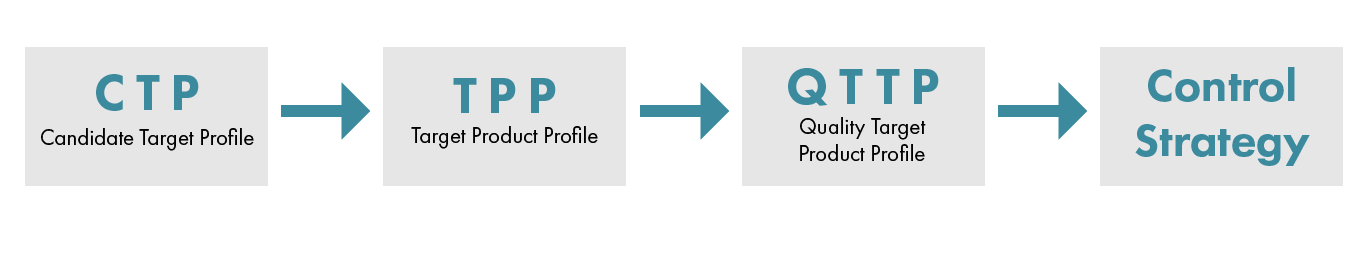 Candidate Target Profile (CTP): The first major decision to make is to identify what the characteristic of a potential candidate looks like. Companies will develop a Candidate Target Profile (CTP), which is a document to help the research team to understand what the product goal is. The CTP may include therapeutic hypothesis, clinical indication, mechanism of action, target population, commercial hypothesis, and many other critical attributes. This approach to document what a desired candidate looks like leads to a formal process for candidates to be nominated and, if approved, advanced for a potential IND submission. The CTP introduces how compounds will be measured against success criteria.
Candidate Target Profile (CTP): The first major decision to make is to identify what the characteristic of a potential candidate looks like. Companies will develop a Candidate Target Profile (CTP), which is a document to help the research team to understand what the product goal is. The CTP may include therapeutic hypothesis, clinical indication, mechanism of action, target population, commercial hypothesis, and many other critical attributes. This approach to document what a desired candidate looks like leads to a formal process for candidates to be nominated and, if approved, advanced for a potential IND submission. The CTP introduces how compounds will be measured against success criteria.
Target Product Profile (TPP): The CTP may not be a requirement for every company, but the Target Product Profile (TPP) usually is, especially since there is a guidance document from the FDA. The TPP is a clear and detailed description of what the product should be (consisting of the method of administration, storage information to start) how it will appear (such as container/closure system, format, and dose) and what it must do (for instance, indications and usage). The TPP is often considered the starting point for the drugs prescribing information, package insert, and product labeling. The type of product will determine what specific information will be needed. CGT will be different than small molecule or large molecule.
Once a candidate nomination has been approved, the CMC Team is formed, and they will use the TPP to develop a project plan with a timeline aligned with the target IND submission, which is most likely a corporate goal. The project plan will need to be detailed enough, with milestones identified, to track the project against the timeline and lead to periodic reporting of progress. The desire to achieve perfection or make something better is part of our continuous improvement DNA. The challenge during this phase of the product lifecycle for CGT teams is determining what is considered “good enough” for an IND submission versus future optimization. This is where clear objectives with a strong data review process are a key success factor.
Quality Target Product Profile (QTTP): The Quality Target Product Profile (QTTP) identifies the critical quality attributes (CQA), such as purity, potency, identify and safety, and other quality attributes with proposed target ranges for the methods identified. This document serves as the baseline of what the new product is expected to deliver. All work by the CMC team is linked to these attributes. It’s important to note that the QTPP is not a material release specification document; although it has a similar focus, the methods and acceptable ranges are most likely aspirational and have not been qualified/validated.
Enhancing Operational Success and Ensuring Product Quality
Having a foundation based on effective communication and documented product expectations enhances operational success. The key documents discussed above are part of an evergreen product process starting with a vision of what a new candidate (potential drug) might look like, to successful nomination, then regulatory submission and approval, followed by product launch, to sunsetting or discontinuation.
A robust knowledge management program ensures that these key documents are not only kept up to date but are easy to find and periodically reviewed, ensuring the quality product continues to be safe and efficacious. Continuous improvement is part of each phase of the product lifecycle, and these documents ensure that lessons learned are documented. When left undocumented, team members in the spirit of innovation – but without the historical product knowledge – may want to try new things, such as different manufacturing processes that have already been demonstrated not to improve the product.
Clarkston is proud to work with many cell and gene therapy companies, from early start ups to commercial, to help them identify and build organizational structures and develop business processes to meet objectives.
Subscribe to Clarkston's Insights
Contributions from Linda Plumley